
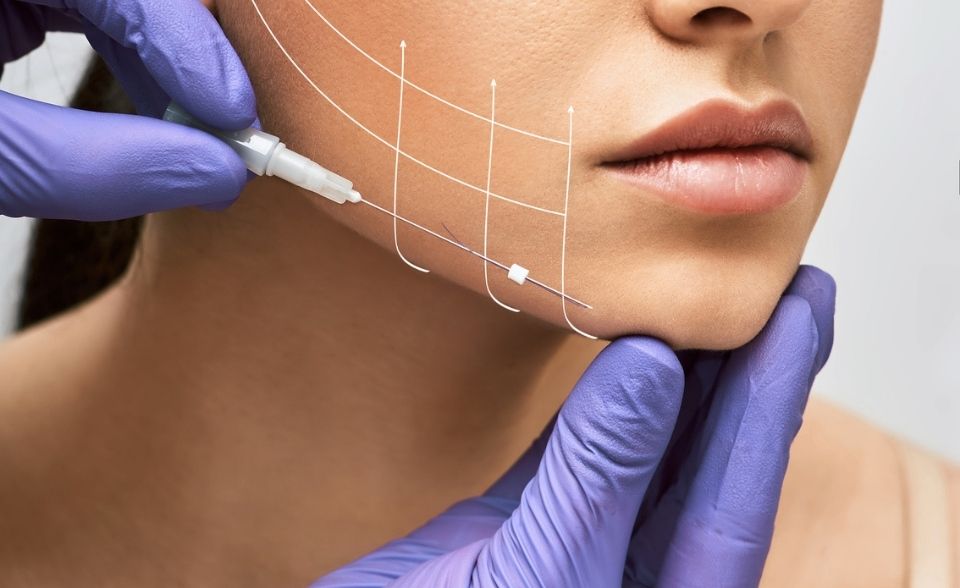
If it is the first time you are seeing us for appearance medicine treatments, or it has been more than 12 months since your last visit, you are required to book a cosmetic consultation with one of our Appearance Medicine Nurses or Doctors.
* The consultation fee with a Nurse is redeemable off your first treatment or used towards any product purchase in clinic.
Botulinum toxin injections are prescription medicine for the treatment of frown lines, horizontal forehead lines and crow’s feet around the eyes. Botulinum toxin injections have risks and benefits. Ask your doctor if botulinum toxin injections are right for you. If you have side effects, see your doctor. You will need to pay for your botulinum toxin injection and clinic fees will apply. For details on precautions & side effects consult your doctor or go to www.medsafe.govt.nz. Botulinum toxin injections last about 4 months and further courses of treatment may be necessary. Should only be administered by trained medical professionals.
Botox® is a prescription medicine containing 100 units of Clostridium Botulinum Type A toxin complex for injection. It is used for the treatment of severe frown lines and associated “crow’s feet” around the eyes. It should be administered only by trained medical professionals. Talk to your specialists about the benefits/risks of this procedure in appearance medicine. Cautions: people with neuro-muscular transmission disorders, presence of infection at site of injection, pregnancy and lactation. Possible side effects include headaches, pain, burning sensation or redness at injection site, temporary local muscle weakness including eyelid droop, decreased sensation and nausea. If you have side effects or concerns, talk to your doctor. A charge applies. Allergan Pharmaceutical, Auckland.
Xeomin® is a Prescription Medicine containing 50, 100 units of incobotulinum Type A, purified Botulinum toxin type A complex for injection. It is used for the treatment of frown lines on the forehead, lateral periorbital lines and horizontal forehead lines in adults. It should be administered only by trained medical professionals. Talk to your specialist about the benefits/risks of this procedure in appearance medicine. Xeomin® treatment lasts about four months and further courses of treatment may be necessary. Cautions: people receiving blood thinning medicines, care at the proposed injection sites, pregnancy and lactation. Possible side effects: headache, pain, swelling or infection at injection site, local muscle weakness including drooping eye lids, lack of feeling & nausea. Treatment last for up to 4 months. You will need to pay for this medicine. Discuss with your specialist if Xeomin® is right for you. For more information or for a copy of CMI please contact the NZ distributor: Merz, Sydney. Distributed by Healthcare Logistics, Auckland.
Profhilo®, containing low & high molecular weight hyaluronic acid, is a Class III medical device for the treatment of the face and body for contours redefinition and laxity remodelling where skin laxity is a problem. Profhilo® has risks and benefits. Do not use with treatments such a laser resurfacing or medium deep skin-peeling. Caution in people on blood thinning medicines. Do not inject into inflamed areas or intravenously or intramuscularly. Possible side effects: pain and swelling at injection site. Please refer to instructions for use, local distributor. Healthcare Logistics, Auckland.
Sunekos 1200 is an implantable medical device that modifies the structure of mature skin, restoring volume, filling wrinkles and folds in the skin and in scar sites. It is suitable for creating a temporary increase in the volume of skin tissue. Sunekos 1200 is a medical device that is sterile, injectable, non-pyrogenic, biocompatible, reabsorbable, made with hyaluronic acid and amino acids. Sunekos Performa is a medical device recommended for the treatment of blemishes and depressions in the skin caused by wrinkles and scars. Sunekos Performa is a sterile resorbable injectable solution which acts as a filler, supporting the restoration of physiological elasticity and temporarily replacing volume by expanding the soft tissues. Sunekos should not be used on patients: with known hypersensitivity to any of its components; presenting with a general infection, inflammatory or infectious cutaneous problems; with history of severe multiple allergies or anaphylactic shock; prone to hypertrophic scars/keloids or streptococcal diseases; in patients presenting with porphyria; under 18 years of age; in pregnancy or lactation; others. See full Instructions for use before prescribing for full safety information, available from www.xytide.co.nz Copyright© 2024. Xytide Biotech Pty Ltd. Always read the label and follow the instructions. This medical device must be administered by a Healthcare Professional. New Zealand Sponsor: AA-Med Pty Ltd Distributed by: Xytide Biotech NZ Pty Ltd (NZBN 9429049668612) C/O Alliott Ltd, Level 2, 142 Broadway, Newmarket, Auckland 1023 NZ. For more information please phone +61 1800 570 036.
PDO threads are made from polydioxanone (PDO), a biocompatible polymer which undergoes complete hydrolytic degradation. The barbs, molded along the thread, anchor into the superficial dynamic fat pads under the skin, to reposition tissue for a non-surgical lift. This can aid in reducing the signs of ageing.